
ISO 13485 International Organization for Standardization ISO 9000 Quality management system Business, Business, blue, label, text png | PNGWing

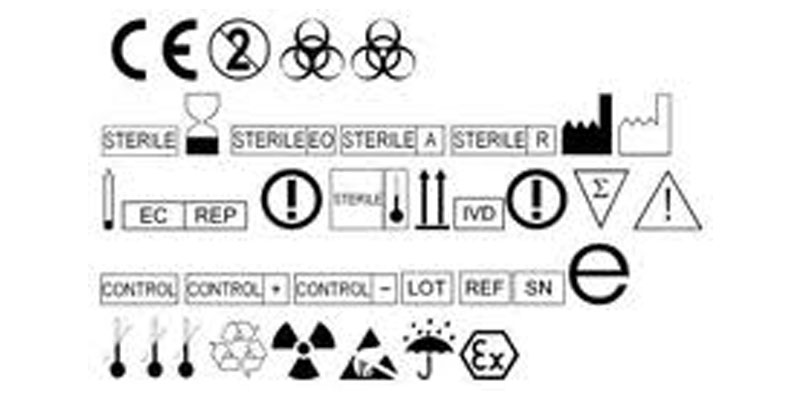
ISO 15223-1:2021(en), Medical devices — Symbols to be used with information to be supplied by the manufacturer — Part 1: General requirements


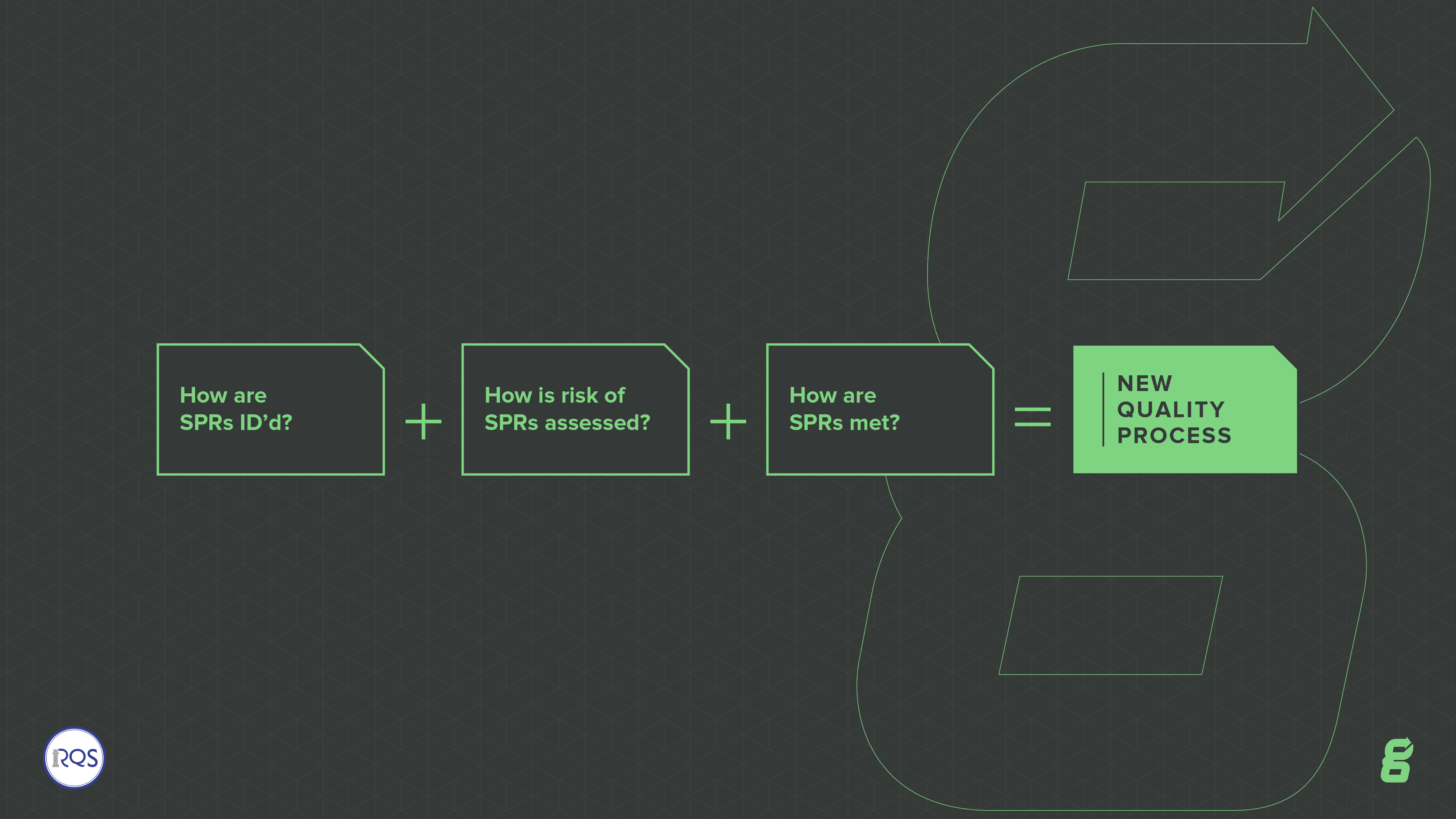

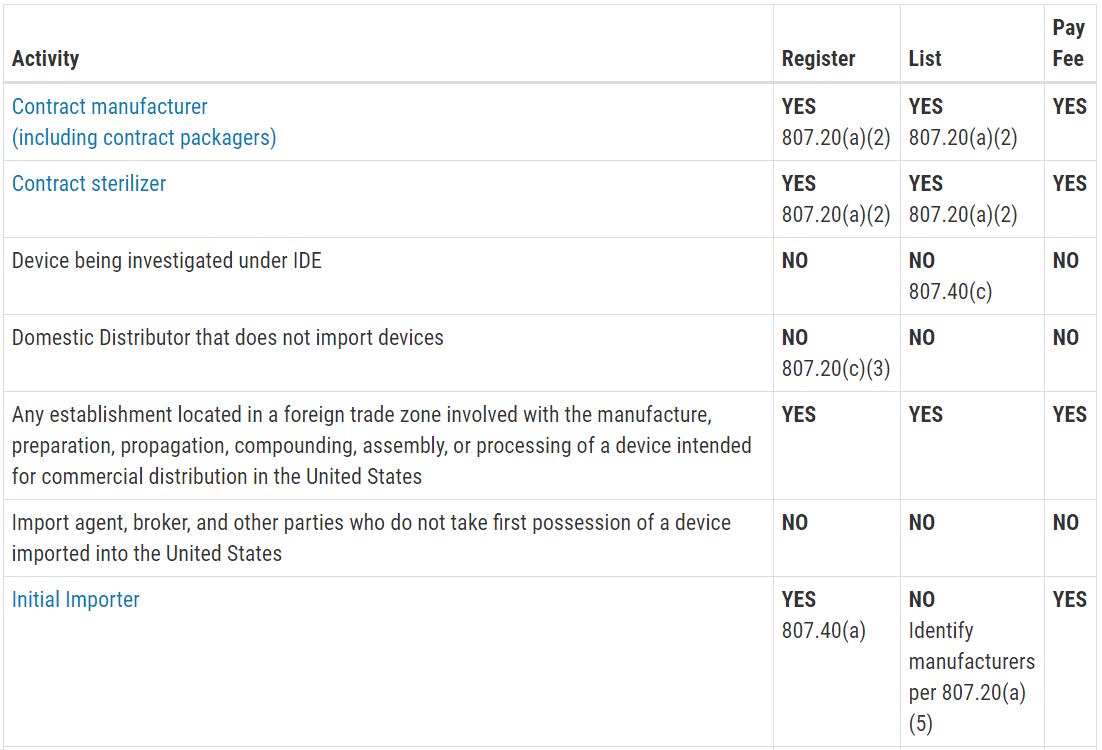

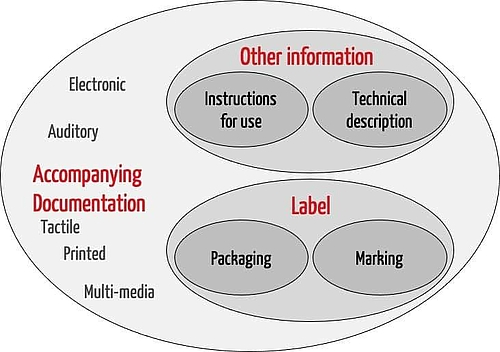





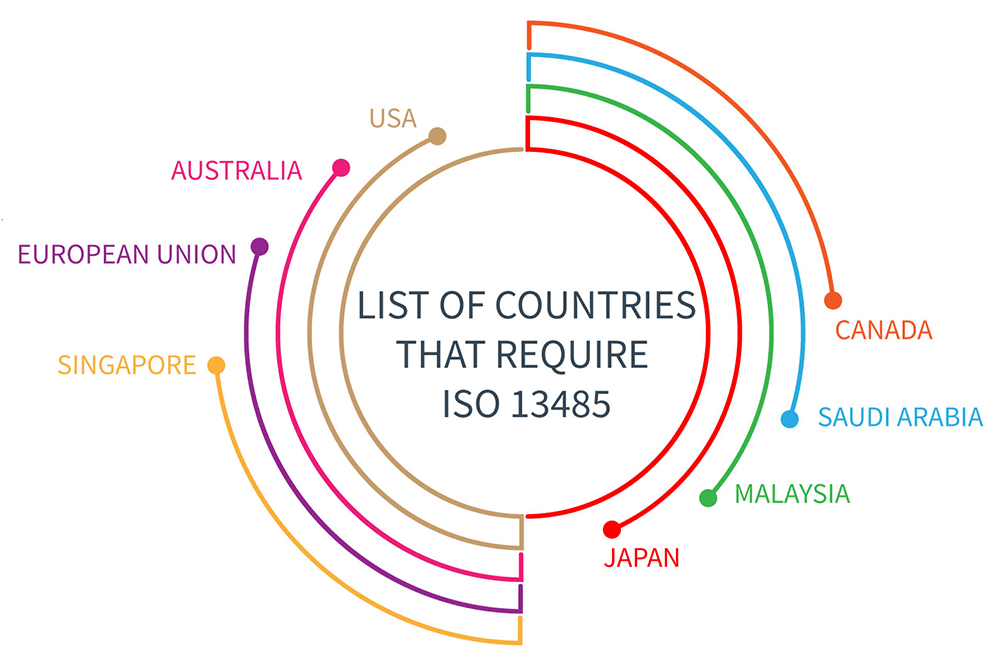




.png.aspx)
![EU MDR vs. MDD: Key differences [Infographic] EU MDR vs. MDD: Key differences [Infographic]](https://advisera.com/wp-content/uploads//sites/14/2020/11/eu-mdr-vs-mdd.png)
